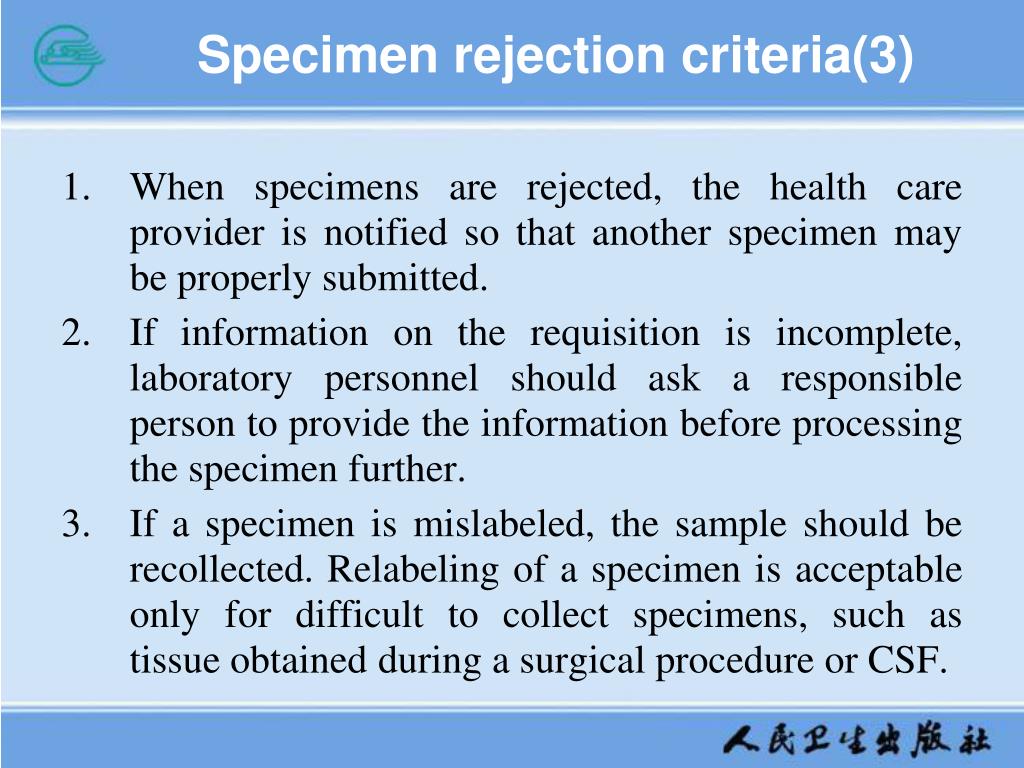
Blood Sample Acceptance And Rejection Criteria . — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. The purpose of this document is to define sample acceptance and rejection criteria. This guideline reflects specimen identification and acceptance criteria that comply with ola. requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable. the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification.
from www.slideserve.com
the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. The purpose of this document is to define sample acceptance and rejection criteria. — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. This guideline reflects specimen identification and acceptance criteria that comply with ola. the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable.
PPT Chapter 26 Specimen Collection and Processing for Microbiology
Blood Sample Acceptance And Rejection Criteria — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. This guideline reflects specimen identification and acceptance criteria that comply with ola. The purpose of this document is to define sample acceptance and rejection criteria. the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable. the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all.
From www.studocu.com
GENSF113 Specimen Rejection Criteria Issue 1 Authorised by Issue Blood Sample Acceptance And Rejection Criteria This guideline reflects specimen identification and acceptance criteria that comply with ola. The purpose of this document is to define sample acceptance and rejection criteria. the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable. before a clinical specimen is accepted, laboratory staff must ensure that the minimum. Blood Sample Acceptance And Rejection Criteria.
From microbeonline.com
Rejection Criteria for Microbiological Specimens • Microbe Online Blood Sample Acceptance And Rejection Criteria the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable. requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the.. Blood Sample Acceptance And Rejection Criteria.
From www.newcastlelaboratories.com
Transfusion Newcastle Laboratories Blood Sample Acceptance And Rejection Criteria requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. This guideline reflects specimen identification and acceptance criteria that comply with ola. before a clinical specimen is accepted, laboratory staff must ensure that. Blood Sample Acceptance And Rejection Criteria.
From www.labpedia.net
Blood Sample Part 3 Types of Blood Samples, Criteria for rejection Blood Sample Acceptance And Rejection Criteria before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. This guideline reflects specimen identification and acceptance criteria that comply with ola. — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. The purpose of this document is to define sample acceptance and rejection criteria.. Blood Sample Acceptance And Rejection Criteria.
From www.labpedia.net
Blood Sample Part 3 Types of Blood Samples, Criteria for rejection Blood Sample Acceptance And Rejection Criteria the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable. — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. requirements. Blood Sample Acceptance And Rejection Criteria.
From www.researchgate.net
(PDF) Pattern of blood specimen rejection at a public clinical laboratory Blood Sample Acceptance And Rejection Criteria The purpose of this document is to define sample acceptance and rejection criteria. This guideline reflects specimen identification and acceptance criteria that comply with ola. requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. the following are the reasons why a sample will be rejected by the microbiology laboratory,. Blood Sample Acceptance And Rejection Criteria.
From www.scribd.com
Sample Acceptance & Rejection Criteria PDF Blood Plasma Medical Blood Sample Acceptance And Rejection Criteria The purpose of this document is to define sample acceptance and rejection criteria. the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable. before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. This guideline reflects specimen identification and acceptance criteria. Blood Sample Acceptance And Rejection Criteria.
From www.labtestpk.com
Hemolysis of Blood Samples Precaution Lab Test Information Blood Sample Acceptance And Rejection Criteria — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. This guideline reflects specimen identification and acceptance criteria that comply with ola. the following are the reasons why a sample will be. Blood Sample Acceptance And Rejection Criteria.
From dxorlaiph.blob.core.windows.net
Specimen Rejection Definition at Violet Lane blog Blood Sample Acceptance And Rejection Criteria The purpose of this document is to define sample acceptance and rejection criteria. the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. requirements for quality and competence’ and the blood. Blood Sample Acceptance And Rejection Criteria.
From www.slideserve.com
PPT Culture of Blood, CSF, and Other Sterile Body Fluids PowerPoint Blood Sample Acceptance And Rejection Criteria requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable. the purpose. Blood Sample Acceptance And Rejection Criteria.
From www.vrogue.co
Microbiology Sample Collection Guidelines And Rejecti vrogue.co Blood Sample Acceptance And Rejection Criteria before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. The purpose of this document is to define sample acceptance and rejection criteria. This guideline reflects specimen identification and acceptance criteria that comply with ola. the purpose of this document is to define sample acceptance and rejection criteria, and to provide. Blood Sample Acceptance And Rejection Criteria.
From www.slideserve.com
PPT Blood Culture PowerPoint Presentation, free download ID9119063 Blood Sample Acceptance And Rejection Criteria requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. The purpose of this document is to define sample acceptance and rejection criteria. before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. This guideline reflects specimen identification and acceptance criteria that comply. Blood Sample Acceptance And Rejection Criteria.
From www.youtube.com
Phlebotomy specimen rejection criteria Blood Talks YouTube Blood Sample Acceptance And Rejection Criteria the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable. requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. the. Blood Sample Acceptance And Rejection Criteria.
From www.slideserve.com
PPT Chapter 26 Specimen Collection and Processing for Microbiology Blood Sample Acceptance And Rejection Criteria the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. requirements for quality and. Blood Sample Acceptance And Rejection Criteria.
From slidetodoc.com
Module 5 Viral Load Specimen Collection and Preparation Blood Sample Acceptance And Rejection Criteria requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. The purpose of this document is to define sample acceptance and rejection criteria. the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. the following are the reasons why a. Blood Sample Acceptance And Rejection Criteria.
From www.semanticscholar.org
Figure 1 from Causes of rejection of blood samples handled in the Blood Sample Acceptance And Rejection Criteria — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. requirements for quality and competence’ and the blood safety and quality regulations 2005 (bsqr) and applies to all. before a clinical specimen is accepted, laboratory staff must ensure that the minimum criteria for sample identification. the purpose of this document. Blood Sample Acceptance And Rejection Criteria.
From www.innoquest.com.sg
Rejection Policy • Innoquest Diagnostics Blood Sample Acceptance And Rejection Criteria the following are the reasons why a sample will be rejected by the microbiology laboratory, in the case of repeatable. The purpose of this document is to define sample acceptance and rejection criteria. the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. — the leading cause of. Blood Sample Acceptance And Rejection Criteria.
From studylib.net
Specimen Rejection Criteria QPCMI06001 Blood Sample Acceptance And Rejection Criteria the purpose of this document is to define sample acceptance and rejection criteria, and to provide guidance for the. This guideline reflects specimen identification and acceptance criteria that comply with ola. — the leading cause of blood specimen rejection in clinical laboratories were clotted specimen (32.23% (95%ci:. The purpose of this document is to define sample acceptance and. Blood Sample Acceptance And Rejection Criteria.